
T cells evolved over millions of years to protect the host from infections through the specific recognition and efficient killing of diseased cells. This concept has been widely used therapeutically over the last decade to treat infections and tumors via the adoptive transfer of naturally occurring, antigen-specific T cells. Recently, the engineering of T cells with antigen-specific T-cell receptors (TCRs) has opened up new possibilities for generating more reliable and versatile “living drugs”. This involves introducing a transgene encoding a TCR of interest into autologous patient-derived lymphocytes ex vivo, thereby redirecting the TCR-engineered T cells against diseased cells once reinfused into the patient. Our goal is to contribute to the accessibility of TCR therapy by developing T-cell manufacturing processes suitable for clinical application, in addition to exploring novel immunotherapeutic approaches. To realize these aims, we will employ innovative CRISPR-Cas9-mediated cell engineering methodologies, alongside high-throughput identification of clinically relevant TCRs and the utilization of informative pre-clinical models.
*dieser Inhalt ist nur auf Englisch verfügbar*
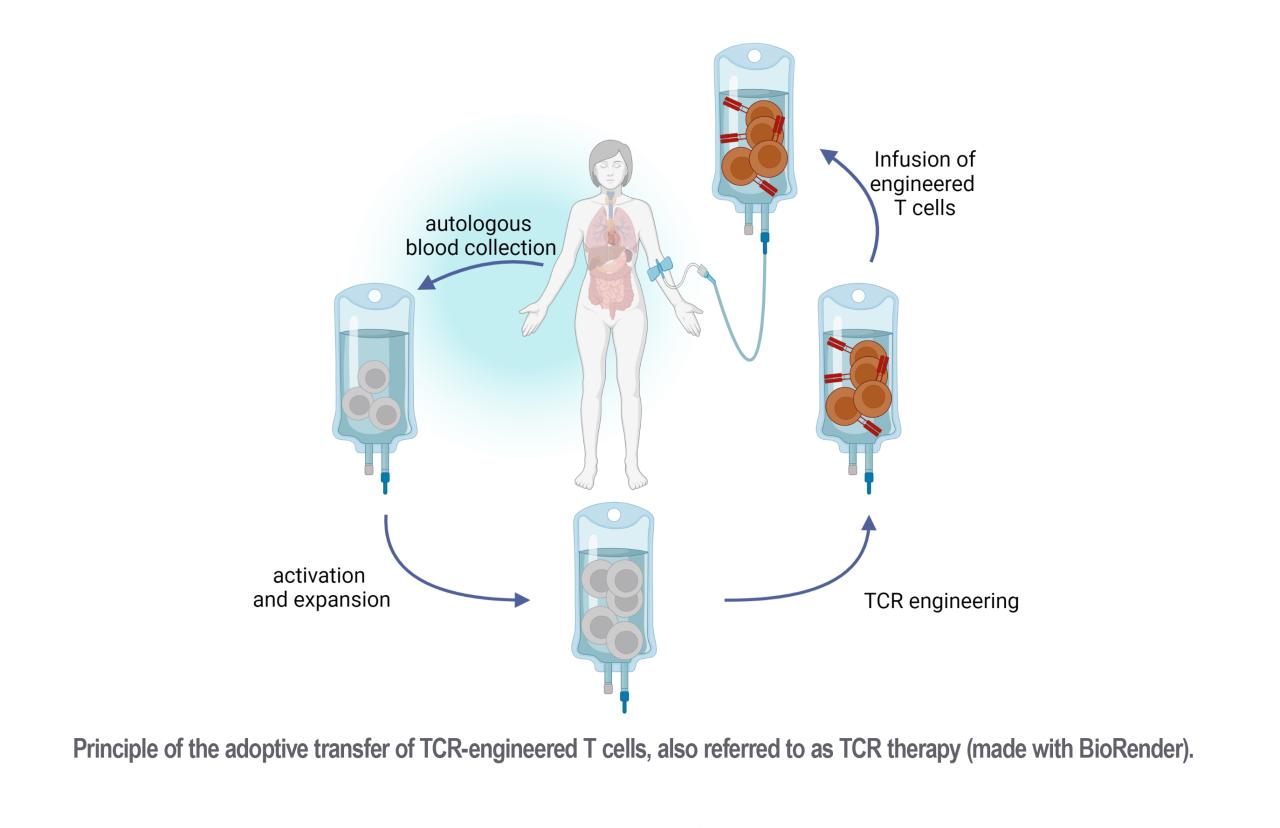
TCR-engineered T cell manufacturing for clinical application
CRISPR-Cas9 technology has revolutionized the field of precise genome editing, including T-cell engineering. Conventional TCR insertion through viral delivery, while highly efficient, can disrupt the regulation of TCR dynamics, affecting T-cell function. In contrast, CRISPR-Cas9 methodologies have been successfully used to integrate a transgenic TCR of desired specificity into the endogenous TCR alpha locus (knock-in) while abrogating the expression of the remaining endogenous TCR beta locus (gene knockout). This innovative approach, called 'orthotopic TCR replacement' (OTR), allows now to replace a T cell's endogenous TCR with a new TCR of interest. The advantages of OTR-engineered T cells over virally engineered ones are noteworthy. OTR-engineered T cells exhibit more consistent surface expression and physiological regulation of the transgenic TCR. This enhanced homogeneity enables the generation of well-defined TCR-transgenic T cell medicinal products with predictable in vivo functions.
Our ultimate goal is to implement this precise and innovative T-cell engineering approach into a GMP-compliant manufacturing process to initiate a Phase I clinical trial for the prevention of CMV reactivation in immunocompromised hosts. Furthermore, our aim to enhance the flexibility and efficiency of manufacturing processes for TCR-T cell products.
Related publications
Development of novel T cell-based immunotherapeutic approaches
The clinical success of cellular therapy with TCR-engineered T cells relies on the infused living drug's ability to activate and sustain a potent immune response against the target cells. T cell activation begins with the recognition of peptides presented by Major Histocompatibility Complex molecules (pMHC) on the target cells through the TCR. The subsequent triggering of the TCR signaling cascade culminates in the production of effector molecules, which are responsible for specific cell killing. However, only a small fraction of the TCR repertoire that recognizes a certain antigen can fully engage a T cell throughout all these steps. Therefore, the identification of TCRs with adequate potency for clinical translation remains a costly, time-consuming and relatively inefficient process.
Our goal is to develop functional screenings and in vitro assays for the high-throughput evaluation of TCR functionality prior re-expression and overall potency of TCR-engineered T cells, respectively. In addition, we aim to explore how novel approaches, such as T cell co-engineering or co-transfer of different T cell products, could enhance the clinical benefits of TCR therapy.
Related publications
D’Ippolito E et al., Int J Mol Sci. 2020